📝 Carbon and its Compounds
Introduction
Carbon is an element. The symbol of carbon is C. It is a non-metal. The name
carbon is derived from the Latin word 'carbo' which means coal.
The earth's crust contains 0.02% carbon and the atmosphere has only 0.03% of
carbon dioxide.
All living things, plants and animals are made up of carbon-based compounds
which are called organic compounds.
Properties of Carbon Compounds
➤ Most carbon compounds are poor conductors of electricity. We can conclude
that the bonding in these compounds does not give rise to any ions.
➤ The carbon compounds have low melting and boiling points as compared to
ionic compounds. We can conclude that the forces of attraction between the
molecules are not very strong.
Electronic Configuration of Carbon.
The atomic number of carbon is 6. Its electronic configuration is 2,
4
In the case of carbon, it has four electrons in its outermost shell and
needs to gain or lose four electrons to attain a noble gas
configuration.
(i) It could gain four electrons forming `C^{4–}` anion. But it would
be difficult for the nucleus with six protons to hold on to ten
electrons, that is, four extra electrons.
(ii) It could lose four electrons forming `C^{4+}` cation. But it
would require a large amount of energy to remove four electrons
leaving behind a carbon cation with six protons in its nucleus
holding on to just two electrons.
Bonding in Carbon - The covalent Bond
Covalent bonds are formed by the sharing of electrons between
two atoms so that both can achieve a completely filled outermost
shell.
The shared electrons ‘belong’ to the outermost shells of both the
atoms and lead to both atoms attaining the noble gas
configuration.
Examples
(i) Hydrogen
The atomic number of hydrogen is 1. Hence hydrogen has one
electron in its K shell and it requires one more electron to
fill the K shell. So two hydrogen atoms share their electrons to
form a molecule of hydrogen, `H_2`. This allows each hydrogen
atom to attain the electronic configuration of the nearest noble
gas, helium, which has two electrons in its K shell. We can
depict this using dots or crosses to represent valence
electrons.
A molecule of hydrogen
The shared pair of electrons is said to constitute a single covalent bond between the two hydrogen atoms. A
single covalent bond is also represented by a line between the two
atoms, as shown in Fig.
The single bond between two hydrogen atoms
In the case of oxygen, we see the formation of a double bond between two oxygen atoms. This is because an atom of oxygen has six electrons in its L shell (the atomic number of oxygen is eight) and it requires two more electrons to complete its octet. So each atom of oxygen shares two electrons with another atom of oxygen to give us the structure shown in Fig.
Double bond between two oxygen atoms
Triple bond between two nitrogen atoms
Covalent bonds.
Such bonds which are formed by the sharing of an electron pair between two atoms are known as covalent bonds.
Characteristics of Covalently Bonded Molecules
Covalently bonded molecules are seen to have strong bonds within the molecule, but intermolecular forces are weak. This gives rise to the low melting and boiling points of these compounds. Since the electrons are shared between atoms and no charged particles are formed, such covalent compounds are generally poor conductors of electricity.
Versatile Nature of Carbon
Catenation
Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. These compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings.
Tetravalency
Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements giving rise to compounds with specific properties.
Saturated compounds
Compounds of carbon, which are linked by only single bonds between the carbon atoms are called saturated compounds. Examples are ethane, propane, etc.
Unsaturated compounds
Compounds of carbon having double or triple bonds between their carbon atoms are called unsaturated compounds. Examples are ethylene, ethene, etc.
Structure of ethane
The structure of ethane arrives in the following steps –
C—C
Three valencies of each carbon atom remain unsatisfied, so each is bonded to three hydrogen atoms giving:
You will see that the valencies of all the atoms are satisfied by single bonds between them. Such carbon compounds are called saturated compounds. These compounds are normally not very reactive.
Electron dot structure of ethane
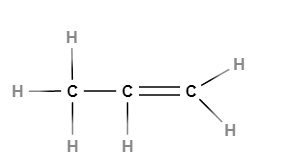
Structure of ethene
Carbon-carbon atoms linked together with a single bond
C—C
We see that one valency per carbon atom remains unsatisfied (Step 2). This can be satisfied only if there is a double bond between the two carbons (Step 3).
Compounds of carbon having double or triple bonds between the carbon atoms are known as unsaturated carbon compounds and they are more reactive than saturated carbon compounds.
Electron dot structure of ethene
Chains, branches and rings
Butane. If we make the carbon ‘skeleton’ with four carbon atoms, we see that two different possible ‘skeletons’ are –
Two possible carbon-skeletons
Filling the remaining valencies with hydrogen gives us –
Complete molecules for two structures with formula `C_4H_10`
Structural Isomers
We see that both these structures have the same formula `C_4H_10`. Such compounds with identical molecular formulas but different structures are called structural isomers.
In addition to straight and branched carbon chains, some compounds have carbon atoms arranged in the form of a ring. For example, cyclohexane has the formula `C_6H_12` and the following structure –
Structure of cyclohexane (a) carbon skeleton (b) complete molecule
Structure of benzene
Hydrocarbons
A Compound made up of hydrogen and carbon only is called hydrocarbon.
(Hydrogen `+` Carbon `=` Hydrocarbon).
Among these, the saturated hydrocarbons are called alkanes. `(C_nH_{2n+2})`. The unsaturated hydrocarbons which contain one or more double bonds are called alkenes. `(C_nH_{2n})`. Those containing one or more triple bonds are called alkynes. `(C_nH_{2n-2})`
Functional Groups
An atom or group of atoms that make a carbon compound reactive and decide its properties is called a functional group.
Carbon also forms bonds with other elements such as halogens, oxygen, nitrogen and sulphur. In a hydrocarbon chain, one or more hydrogens can be replaced by these elements, such that the valency of carbon remains satisfied. In such compounds, the element replacing hydrogen is referred to as a heteroatom. These heteroatoms and the group containing these confer specific properties to the compound, regardless of the length and nature of the carbon chain and hence are called functional groups.
Some functional groups in carbon compounds
Naming of Hydrocarbons
Organic compounds have two names:
- Common names
- Official names (IUPAC names)
IUPAC 👉 International Union of Pure and Applied Chemistry
In order to name hydrocarbons by the IUPAC method, we should remember the following points:
1. The number of carbon atoms in a hydrocarbon is indicated by using the following stems:
One carbon atom 👉 Meth
Two carbon atoms 👉 Eth
Three carbon atoms 👉 Prop
Four carbon atoms 👉 But
Five carbon atoms 👉 Pent
Six carbon atoms 👉 Hex
Seven carbons atoms 👉 Hept
Eight carbons atoms 👉 Oct
Nine carbons atoms 👉 Non
Ten carbon atoms 👉 Dec
2. A saturated hydrocarbon containing a single bond is indicated by writing the word 'ane' after the stem
3. A unsaturated hydrocarbon containing a double bond is indicated by writing the word 'ene' after the stem.
4. A unsaturated hydrocarbon containing a triple bond is indicated by writing the word 'yne' after the stem.
Naming of Saturated Hydrocarbons
1. Naming of `CH_4`
One carbon atom 👉 Meth
This compound has all single bond, so it is saturated. The saturated hydrocarbon is indicated by the ending 'ane'.
IUPAC name 👉 Meth `+` ane `=` Methane
Common name 👉 Methane
2. Naming of `C_2H_6`
Two carbon atoms 👉 Eth
This hydrocarbon has all single bond, so it is saturated. The saturated hydrocarbon is indicated by the ending 'ane'.
IUPAC name 👉 Eth`+` ane `=` Ethane
Common name 👉 Ethane
work in progress
Naming of Unsaturated Hydrocarbons Containing a Double Bond
1. Naming of `C_2H_4`
 |
| IUPAC Name: Ethene Common Name: Ethylene |
This hydrocarbon contains 2 carbon atoms which are indicated by writing 'eth'. This hydrocarbon has a carbon-carbon double bond so it is unsaturated. The double bond is indicated by 'ene'.
eth `+` ene `=` ethene. IUPAC Name: Ethene, Common Name: Ethylene
2. Naming of `C_3H_6`
prop `+` ene `=` propene. IUPAC Name: propene, Common Name: Propylene
Naming of Unsaturated Hydrocarbons Containing a Triple Bond
1. Naming of `C_2H_2`
Work in progress
Homologous Series
A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by `CH_2` group.
Characteristics of a Homologous Series
1. All the members of a homologous series can be represented by the same general formula. For example, all the members of the alkane series can be represented by the general formula `C_nH_{2n+ 2}`.
2. Any two adjacent homologues differ by `CH_2`.
3. The difference in the molecular masses of any two adjacent homologues is 14 u. For example, the molecular mass of the methane `(CH_4)` is 16u, and that of its next higher homologue ethane is `(C_2H_6)` is 30u. So the difference in the molecular masses of ethane and methane is 30 - 16 = 14u.
4. All the compounds of a homologue series show similar chemical properties.
5. The members of the homologous series show a gradual change in their physical properties with an increase in molecular mass. For example, in the alkane series as the number of carbon atoms per molecule increases, the melting points, boiling points and densities of its members increase gradually.
Work in progress
Nomenclature of organic compounds
Haloalkanes
When one hydrogen atom of an alkane is replaced by a halogen atom, we get haloalkane. For example, when one hydrogen atom of methane `(CH_4)` is replaced by a chlorine atom, we get chloromethane `(CH_3Cl)`. The general formula of haloalkane is `C_nH_{2n+1}-X`.
Examples: Chloroethane `(C_2H_5Cl)`, Chloropropane `(C_3H_7Cl)`.
Alcohols
Alcohols are the organic compounds containing hydroxyl group `(-OH)` attached to a carbon atom. By replacing one hydrogen atom of methane `(CH_4)` with a hydroxyl group we get alcohol called methanol `(CH_3OH)`. The general formula of homologous series of alcohol is `C_nH_{2n+1}-OH`.
Aldehydes
Aldehydes are the organic compound containing an aldehyde group `(-CHO)` attached to the carbon atom. The general formula of aldehyde is `C_nH_{2n}O`.
Examples:
Methanal `(HCHO)`
Ethanal `(CH_3CHO)`
Propanal `(CH_3CH_2CHO)`
Butanal `(CH_3CH_2CH_2CHO)`
Ketones
Ketones are the carbon compounds containing the ketone group `-CO-`. Please note that a ketone group always occurs in the middle of the carbon chain, so a ketone must contain at least three carbon in its molecule. The general formula is `C_nH_{2n}O`
Examples:
Propanone `(CH_3COCH_3)`
Butanone `(CH_3COCH_2CH_3)`
Pentanone `(CH_3COCH_2CH_2CH_3)`
Carboxylic acids
The carbon compounds containing carboxylic acid group `(-COOH)` are called carboxylic acid. The general formula is `R-COOH` (R is an alkyl group like methyl, ethyl).
Examples:
Methanoic acid `(HCOOH)`
Ethanoic acid `(CH_3COOH)`
Propenoic acid `(C_2H_5COOH)`
Butanoic acid `(C_3H_7COOH)`
Chemical Properties of Carbon Compounds
The chemical properties which are going to study here are combustion reactions, substitution reactions, and addition reactions. Combustion reactions occur in all types of hydrocarbons (saturated as well as unsaturated), substitution reactions are given by only saturated hydrocarbons whereas addition reactions are given by only unsaturated hydrocarbons (alkenes & alkynes).
1. Combustion
The process of burning a carbon compound in the air to give carbon dioxide, water, heat and light, is known as combustion.
Most carbon compounds also release a large amount of heat and light on burning.
(i) `C + O_2 rightarrow CO_2 +` heat and light
(ii) `CH_4 + O_2 rightarrow CO_2 + H_2O +` heat and light
(iii) `CH_3CH_2OH + O_2 rightarrow CO_2 + H_2O +` heat and light
Saturated hydrocarbons will generally give a clean flame while unsaturated carbon compounds will give a yellow flame with lots of black smoke. This results in a sooty deposit on the metal plate.
The cooking gas (LPG) which we use in our homes is mainly an alkane called butane `(C_4H_10)`.






















👍👍👍👍
ReplyDeleteHelpful notes 📝☺😇👍
ReplyDeleteNice notes 🙂🙂
ReplyDeleteThank you
Deletevery understanding notice sir jii
ReplyDeleteThanks for notice o:-)💯💯👍👍👍🪙
👍👍👍👍
ReplyDelete❣️❣️ well done ..........🤗🤗
ReplyDelete